Q-Las
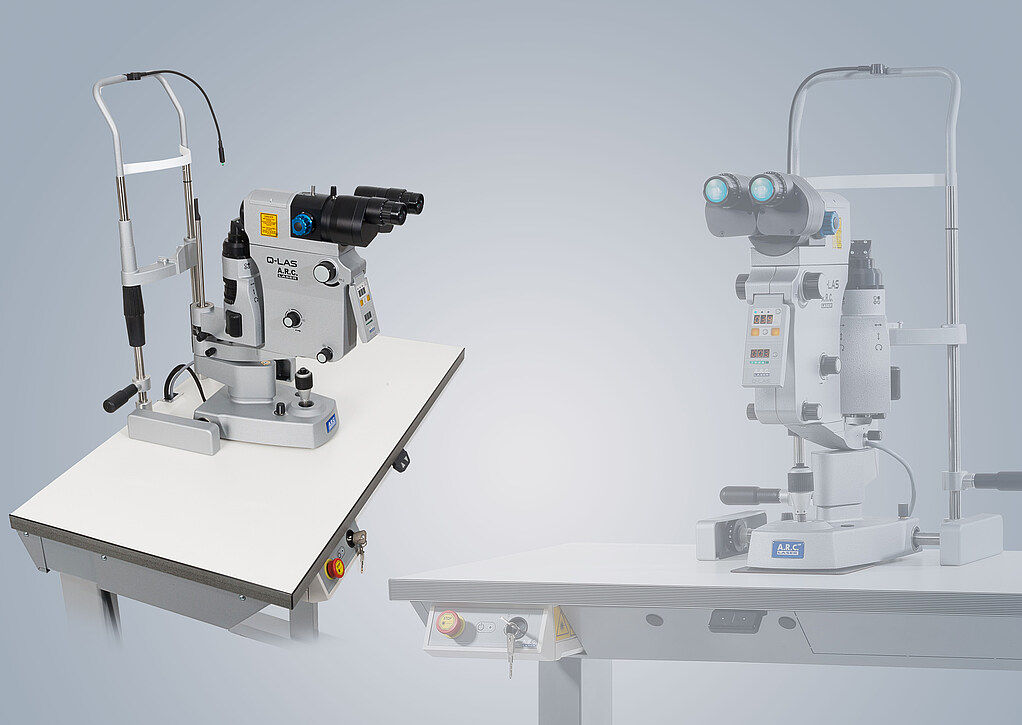
Q-Las
the Nd:YAG laser for your practice
The Q-LAS. It combines economic efficiency, ergonomics and longevity in a very innovative way. Energy settings from 0.5 to 10 mJ can be made via the intuitive operating concept and it is also possible to switch between Burst-Mode levels at the touch of a button. This gives you up to 35 mJ at your disposal.
- Superior focusing via a 2-divided aiming beam
- 3-fold Burst-Mode
- Laser beam and slit illumination are coaxial
- Integrated Clear-View-Filter-Technology
- Treatment parameters always in view with the optional head-up display
Combination systems
Q-LAS + PCL5 ZL
- with classic YAG-slit lamp
- Illumination beam path via prism
Q-LAS + PCL5 SHL
- Light source at the top of the slit projector
- highest optical quality
Combination system VARIO
The Q-Las can be combined with the Classic retina laser. Furthermore, the Q-Las is available with two different types of slit lamp.

Technical data
| Laser | Nd:YAG |
| Display | 7-segment Display, Idicator LED |
| Wavelength | 1064 nm |
| Pulse length | 4 ns |
| Output | 0.5-10 mJ, continuous |
| Spot diameter in air | < 10 µm |
| Angle laser beam | 16° |
| Burst Mode | 1-2-3 pulses |
| Repetition frequency | 4 Hz, independent on Burst Mode |
| Mode Structure | Gaussian |
| Focus shift | 3-stage – 0/150/300 µ – posterior |
| Aiming beam | Red / 2-beam |
| Power supply | 100 – 240 V AC, 50 – 60 Hz |
| Dimensions | 43 (H) x 33 (B) x 50 (T) cm only Laser; 130 (HH) x 86 (B) x 57 (T) cm incl. table |
| Weight | 12,6 kg laser integrated in slit lamp, 44 kg with tableh |
| Cooling | Air |
Changes to equipment and specifications to provide technical progress are carried out automatically.
The TruBlue Wolf 445 and the FOX 980 lasers are cleared by the U.S. Food and Drug Administration (FDA) for incision, excision, vaporization, ablation, hemostasis, and coagulation of soft tissue. Other products, indications, or clinical applications described on this website are not cleared by the FDA for use in the United States. Information presented is intended for educational purposes only and does not constitute medical advice. For details regarding product availability, regulatory status, and indications for use, please contact A.R.C.

