SLT-Laser CITO 532
The SLT-Laser CITO 532
Revised from scratch:
Modern technology “Made in Germany” and convincing technical refinements redefine the SLT. After two years of development a unique laser system for gentle glaucoma therapy has emerged which we are proud of: The CITO 532 has a theoretically unlimited lifetime, so you can work stably and without energy loss over its entire life cycle. The CITO has no heat losses on the housing and no harmful UV-light on the cavity.

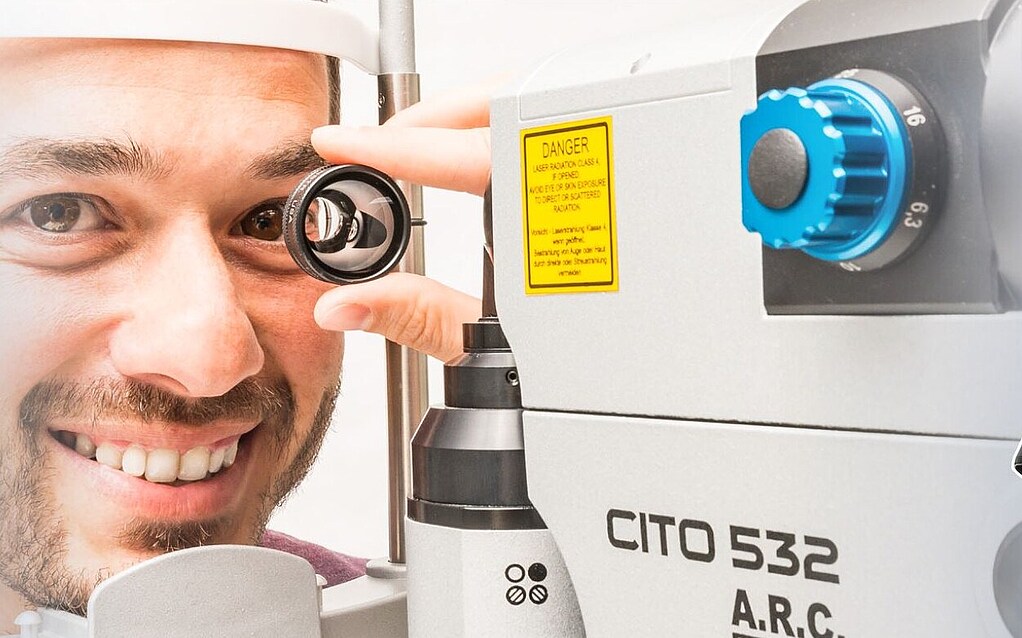
Buying a CITO 532 means making no compromises.
Less pain – Less stress
World-leading, strong and durable: This is how the CITO 532 presents itself to you. Convince yourself of the unique innovations such as Quick-Repetition for a fast frequency or the large touchscreen that allows you to always keep an eye on the selected treatment energy.
Want more?
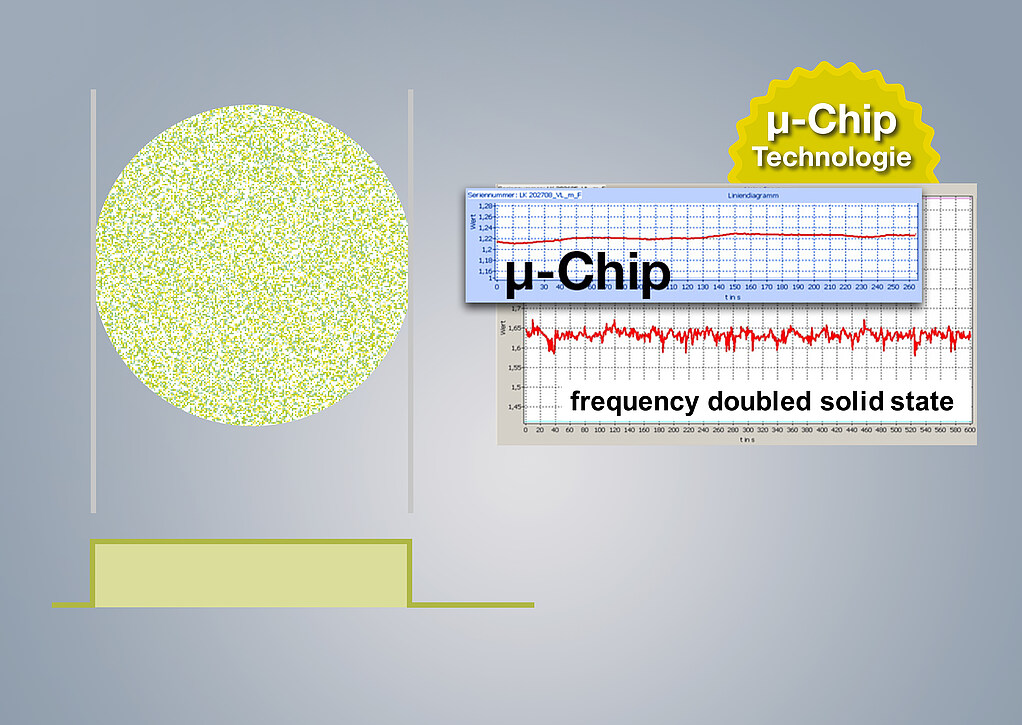
Fastest repetition frequency worldwide
Other SLT systems are based on flash lamp technology. The resulting active beam depends on charging cycles of the capacitors, which is why they are so slow. The modern µ-chip SLT from A.R.C. on the other hand convinces by:
- Quick pulse sequences
- Perfect stain quality
- Temperature stability
SLT laser:
- Quick Refresh ~10Hz Rep-Rate
- TouchScreen
Technical data
| Laser | Q-switched frequency doubled Nd:YAG |
| Display | |
| Wavelength | 532 nm |
| Output power | 0.2 to 2.0 mJ, quasi continuous |
| Spot size | 400 μm in target beam focus |
| Repetition rate, pulses | >10 Hz |
| Pulse width | 3 ns |
| Aiming beam | red 635 nm / 1 mW, adjustable |
| Doctor protection filters | permanent |
| Laser exit angle | 3,2° |
| Laser transmission | Coupling in column lighting |
| Design | Mid-middle with the microscope |
| Stand area | 0.5 m² |
| Connection values | 100 to 240V 50/60 Hz, 600 Watts |
| Weight | 53 kg with slit lamp |
| Laser class | 3b 532 nm, E=2.5 mJ aiming beam: 635 nm, P<5 mW |
Deviations from the features described or illustrated above are possible. Please always inform yourself about the current status before purchasing. Changes and errors reserved.
The TruBlue Wolf 445 and the FOX 980 lasers are cleared by the U.S. Food and Drug Administration (FDA) for incision, excision, vaporization, ablation, hemostasis, and coagulation of soft tissue. Other products, indications, or clinical applications described on this website are not cleared by the FDA for use in the United States. Information presented is intended for educational purposes only and does not constitute medical advice. For details regarding product availability, regulatory status, and indications for use, please contact A.R.C.

